1

Anti-Tubulin alpha chain (polyclonal antibodies)
AS10 680 | Clonality: Polyclonal | Host: Rabbit | Reactivity: A. thaliana, C. reinhardii, E. gracilis, H. vulgare, O. sativa, Setaria italica, Zea mays
- Data sheet
-
- Product Info
-
Immunogen: KLH-conjugated peptide derived from available tubulin alpha chain sequences including Arabidopsis thaliana tubulin alpha-1-chain P11139(At1g64740), alpha-2/alpha-4 chain B9DGT7(At1g50010), alpha-5 chain B9DHQ0(At5g19780), alpha-6-chain P29511(At4g14960)
Peptide used to elict this antibody is not present in tubulin beta.Host: Rabbit Clonality: Polyclonal Purity: Serum Format: Lyophilized Quantity: 50 µl Reconstitution: For reconstitution add 50 µl of sterile water Storage: Store lyophilized/reconstituted at -20°C; once reconstituted make aliquots to avoid repeated freeze-thaw cycles. Please remember to spin the tubes briefly prior to opening them to avoid any losses that might occur from material adhering to the cap or sides of the tube. Tested applications: Immunofluorescence (IF), Western blot (WB) Recommended dilution: 1 : 500 (IF), 1 : 1000 (WB) Expected | apparent MW: 49 | 52 kDa (Arabidopsis thaliana)
- Reactivity
-
Confirmed reactivity: Arabidopsis thaliana, Chlamydomonas reinhardii, Euglena gracilis, Hordeum vulgare, Oryza sativa, Setaria italica, Zea mays
Predicted reactivity: Brassica napus, Chlorella vulgaris, Chlorella variabilis, Cucumis sativus, Eragrostis tef, Euglena gracilis, Glycine max, Micromonoas pusilla, Nannochloropsis gaditana, Ostreococcus lucimarinus, Pisum sativum, Physcomitrium patens, Picea sitchensis, Populus trichocarpa, Solanum tuberosum, Sorghum bicolor, Ricinus communis, Triticum aestivum, Vigna radiata, Vitis vinifera
Species of your interest not listed? Contact usNot reactive in: No confirmed exceptions from predicted reactivity are currently known - Application Examples
-
Western Blot

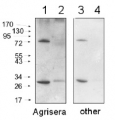
1 - 20 μg of Arabidopsis thaliana whole rosette leaves extract
2 - 10 μg of Arabidopsis thaliana whole rosette leaves extract
3 - 5 μg of Arabidopsis thaliana whole rosette leaves extract20, 10 and 5 μg of total protein from Arabidopsis thaliana rosette leaves extracted with extraction buffer 100 mm Tris-HCl, pH 8.5, 4% [w/v] SDS, 2% [v/v] β-mercapthoethanol, and 2 mm phenylmethylsulfonyl fluoride and separated on 10% SDS-PAGE and blotted 1h to PVDF. Blots were blocked with 1% non-fat milk in TBS for 1 h at room temperature with agitation. Membranes were incubated in the primary antibody at a dilution 1:5000 (A) or 1:1000 (B) in 1% non-fat milk in TBS at 4°C overnight. Then, membranes were washed 3 Times for 15 min in TBS at RT with agitation. Membranes were incubated in secondary antibody (Anti-rabbit IgG horse radish peroxidase conjugated from Agrisera AS09 602) diluted to 1: 25 000 in 1% non-fat milk in TBS for 1h at RT with agitation. The blots were washed as above and developed for 5 min with ECL (Agrisera ECL Bright AS16 ECL-N) according to the manufacturer's instructions. Exposure time was 2 seconds.
Courtesy of Dr. Agnieszka Zienkiewicz, Centre for Modern Interdisciplinary Technologies, Nicolaus Copernicus University, Toruń, Poland
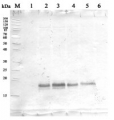
5 µg of total protein from Arabidopsis thaliana (1), Hordeum vulgare (2), Zea mays (3) extracted with Agrisera PEB extraction buffer were separated on 4-12 % SDS-PAGE and blotted 1h to PVDF. Blots were blocked with for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 10 000 for 1h at RT with agitation. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated, from Agrisera AS09 602 ) diluted to 1:25 000 in for 1h at RT with agitation. The blot was washed as above and developed for 5 min with ECL Advance according to the manufacturers instructions (GE Health care). Exposure time was 120 seconds.

Total protein from either rice embryos or rice protoplasts extracted with buffer containing 60mM Tris-HCl (pH8.0), 2% SDS(w/v), 15% Sucrose (w/v) and protease inhibitor 1X were separated on 12 % SDS-PAGE and blotted 1h to PVDF. Blots were blocked with skimmed milk containing TBS 1X for 1h at room temperature (RT) with agitation. Blot was incubated in the primary antibody at a dilution of 1: 1 000 overnight at 4 degree with shaking about 40 rpm. The antibody solution was decanted and the blot was rinsed briefly twice, then washed once for 15 min and 3 times for 5 min in TBS-T at RT with agitation. Blot was incubated in secondary antibody (anti-rabbit IgG horse radish peroxidase conjugated) diluted to 1:20 000 in for 1h at RT with agitation. The blot was washed as above and developed for 5 min with ECL according to the manufacturers instructions. Exposure time was 10 seconds.
Courtesy of Ho Viet The, PhD Student, Scuola Superiore Sant'Anna, Pisa, ItalyImmunolocalization

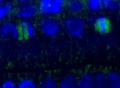
Tubulin alpha localization in roots of Arabidopsis thaliana. Tubulin alpha (red), nucleus (DAPI white). Plant material has been fixed in para-formaldehyde for 30 minutes. Tissue cleaning has been performed before immunolocalization. Primary antibodies: Agrisera anti-tubulin alpha 1: 500. Secondary antibody: goat anti-rabbit IgG Alexa conjugated (red color), dilution 1: 500. Scale bar – 10 µm.
Courtesy Dr. Taras Pasternak, Freiburg University, Germany
Application examples: 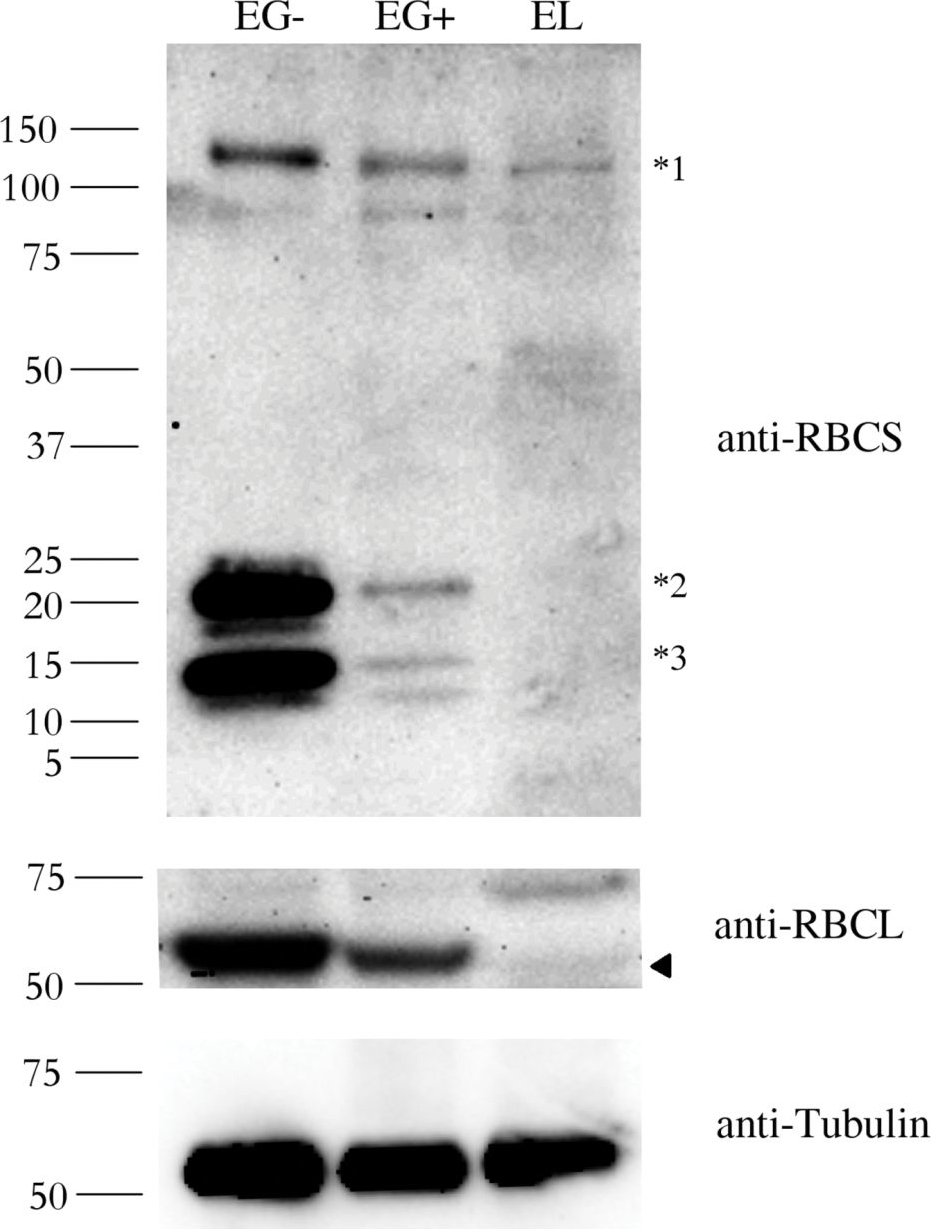
Reactant: Euglena
Application: Western Blotting
Pudmed ID: 27391690
Journal: PLoS One
Figure Number: 2A
Published Date: 2016-07-09
First Author: Záhonová, K., Füssy, Z., et al.
Impact Factor: 2.942
Open PublicationAbundance of the RBCS and RBCL proteins in Euglena gracilis and Euglena longa.Protein immunodetection was performed using anti-RBCS, anti-RBCL, and anti-Tubulin antibodies. Three bands with different molecular weights were observed in anti-RBCS immunoblotting. The ~130 kDa band (marked *1) corresponds to polyprotein synthesized in the nucleus. The ~15 kDa band (marked *3) corresponds to the processed monomer after cleavage of the signal sequence and excision of decapeptides. The ~22 kDa band (marked *2) possibly corresponds to a monomer still attached to the transit peptide. The identity of the RBCL protein (arrowhead in the anti-RBCL panel) was confirmed by mass-spectrometry. Tubulin served as a loading control. Molecular weights in kDa are indicated on the left. EG-, E. gracilis cultivated photosynthetically (without ethanol); EG+, E. gracilis cultivated mixotrophically (with ethanol); EL, E. longa.
Reactant: Euglena
Application: Western Blotting
Pudmed ID: 27391690
Journal: PLoS One
Figure Number: 4A,B,C
Published Date: 2016-07-09
First Author: Záhonová, K., Füssy, Z., et al.
Impact Factor: 2.942
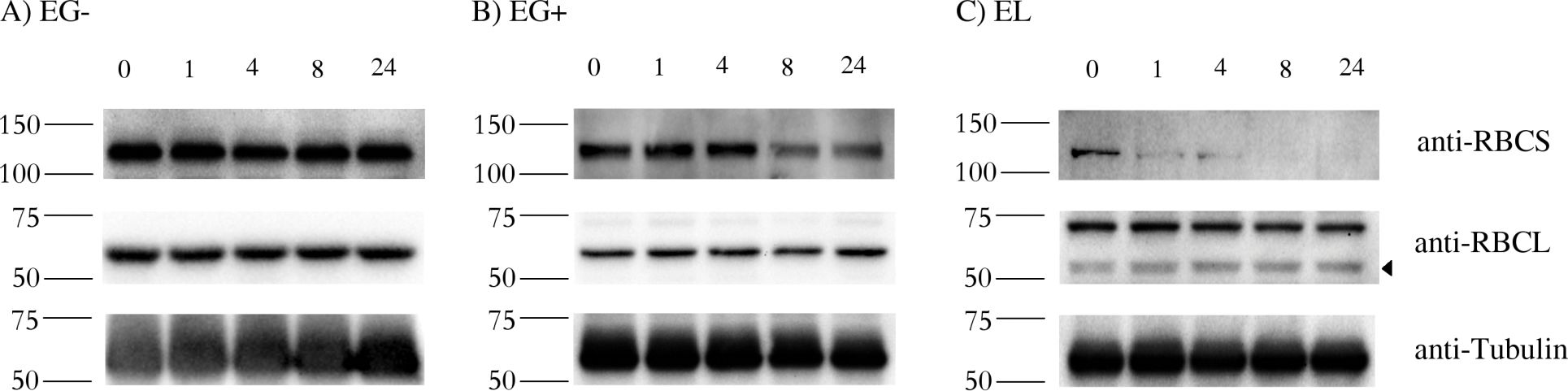
Open PublicationStability of RBCS and RBCL proteins in Euglena gracilis and Euglena longa.Cell cultures were treated with 20 ?g/ml of cycloheximide, aliquots were taken at 0, 1, 4, 8, and 24 h post treatment, and analyzed by western blotting using anti-RBCS, anti-RBCL and anti-Tubulin antibodies. Molecular weights (in kDa) are indicated on the left of each panel. The identity of the RBCL protein (arrowhead in the anti-RBCL panel) was confirmed by mass-spectrometry. Tubulin served as a loading control. Cultivation conditions and species are denoted as in Fig 2.
- Additional Information
-
Additional information (application): Metal induced stress affected the expression of tubulin, and that therefore, this protein cannot be used as a loading control under that type of conditions. More information can be found here. - Background
-
Background: Tubulin alpha (TUA) together with beta tubulin is making up microtubules.
- Product Citations
-
Selected references: Seed priming with NaCl helps to improve tissue tolerance, potassium retention ability of plants, and protects the photosynthetic ability in two different legumes, chickpea and lentil, under salt stress. Planta. 2023 May 8;257(6):111. doi: 10.1007/s00425-023-04150-y.
Zhao et al (2023) Maize tubulin folding cofactor B is required for cell division and cell growth through modulating microtubule homeostasis.
Ye, Zhou, Zhu, et al. (2022) Inhibition of shoot-expressed NRT1.1 improves reutilization of apoplastic iron under iron-deficient conditions. Plant J. 2022;112(2):549-564. doi:10.1111/tpj.15967
Graf et al. (2024). D6PK plasma membrane polarity requires a repeated CXX (X) P motif and PDK1-dependent phosphorylation. Nat Plants. 2024 Feb;10(2):300-314.
Kafri et al. (2023). Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery. Cell. 2023 Dec 7;186(25):5638-5655.e25.doi: 10.1016/j.cell.2023.11.007.
Kumar et al. (2022). Proteomic dissection of rice cytoskeleton reveals the dominance of microtubule and microfilament proteins, and novel components in the cytoskeleton-bound polysome, Plant Physiology and Biochemistry, Volume 170,2022,Pages 75-86,ISSN 0981-9428, https://doi.org/10.1016/j.plaphy.2021.11.037.
Gu et al. (2021) A Lipid Bodies-Associated Galactosyl Hydrolase Is Involved in Triacylglycerol Biosynthesis and Galactolipid Turnover in the Unicellular Green Alga Chlamydomonas reinhardtii
Sakuraba at al. (2020). Multilayered regulation of membrane-bound ONAC054 is essential for abscisic acid-induced leaf senescence in rice. Plant Cell. 2020 Jan 6. pii: tpc.00569.2019. doi: 10.1105/tpc.19.00569.
Roustan et al. (2020). Protein sorting into protein bodies during barley endosperm development is putatively regulated by cytoskeleton members, MVBs and the HvSNF7s. Sci Rep. 2020 Feb 5;10(1):1864. doi: 10.1038/s41598-020-58740-x.
Roustan et al. (2020). Protein sorting into protein bodies during barley endosperm development is putatively regulated by cytoskeleton members, MVBs and the HvSNF7s. Sci Rep. 2020 Feb 5;10(1):1864. doi: 10.1038/s41598-020-58740-x.
Upadhyaya and Jagadeeshwar Rao (2019). Reciprocal regulation of photosynthesis and mitochondrial respiration by TOR kinase in Chlamydomonas reinhardtii. Plant Direct Volume 3, Issue 11.
Li et al. (2019). A genome-wide algal mutant library and functional screen identifies genes required for eukaryotic photosynthesis. Nat Genet. 2019 Apr;51(4):627-635. doi: 10.1038/s41588-019-0370-6.
Pan et al. (2018). Comparative proteomic investigation of drought responses in foxtail millet. BMC Plant Biol. 2018 Nov 29;18(1):315. doi: 10.1186/s12870-018-1533-9.
Nasir et al. (2018). Identification of a flagellar protein implicated in the gravitaxis in the flagellate Euglena gracilis. Sci Rep. 2018 May 15;8(1):7605. doi: 10.1038/s41598-018-26046-8.
Kwon et al. (2018). AtCAP2 is crucial for lytic vacuole biogenesis during germination by positively regulating vacuolar protein trafficking. Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1675-E1683. doi: 10.1073/pnas.1717204115.
Ho et al. (2017). A calcineurin B-like protein participates in low oxygen signalling in rice. CSIRO PUBLISHING Functional Plant Biology.
Wei et al. (2017). Light Intensity is Important for Hydrogen Production in NaHSO3-Treated Chlamydomonas reinhardtii. Plant Cell Physiol. 2017 Mar 1;58(3):451-457. doi: 10.1093/pcp/pcw216.
Nasir (2016). Analysis of signal transduction chains of gravity and light sensing in Euglena gracilis. Doctoral Thesis, urn:nbn:de:bvb:29-opus4-79185.
Armbruster et al. (2014). Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat Commun. 2014 Nov 13;5:5439. doi: 10.1038/ncomms6439
Juszczak et al. (2012). Natural genetic variation in the expression regulation of the chloroplast antioxidant system among Arabidopsis thaliana accessions. Physiol. Plant. - Protocols
-
Agrisera Western Blot protocol and video tutorials
Protocols to work with plant and algal protein extracts - Reviews:
-
This product doesn't have any reviews.